- LIFESTYLE
- FASHION
- FOOD
- ENTERTAINMENT
- EVENTS
- CULTURE
- VIDEOS
- WEB STORIES
- GALLERIES
- GADGETS
- CAR & BIKE
- SOCIETY
- TRAVEL
- NORTH EAST
- INDULGE CONNECT

The US Food and Drug Administration (FDA) has approved the sleep apnoea detection feature for the Apple Watch Series 10, Series 9, and Watch Ultra 2.
This approval comes just ahead of the Apple Watch Series 10's release on September 20.
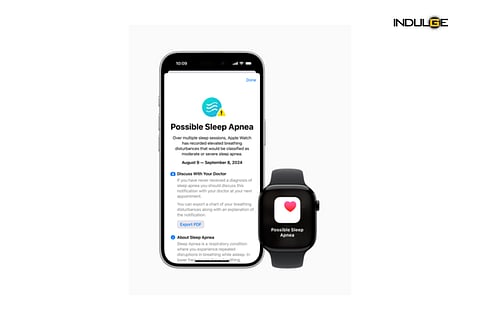
The feature, announced alongside the iPhone 16 last week, will be included in the watchOS 11 update. It uses software algorithms to analyse sensor data and provide a risk assessment for sleep apnoea, but it is not designed to offer a standalone diagnosis or replace traditional diagnostic methods such as polysomnography. The FDA clarified that the device is intended to assist in identifying potential issues and prompting users to seek formal medical evaluation.
The sleep apnoea detection, a first for the Apple Watch, will be available on Series 9, Series 10, and Watch Ultra 2 models. According to Apple, the detection algorithm was developed using advanced machine learning techniques and a comprehensive data set from clinical sleep apnoea tests.
The new metric will monitor users' sleep, analyse patterns, and alert them if signs of apnoea are detected. The feature relies on the watch's accelerometer to identify small wrist movements linked to disruptions in breathing during sleep, notifying users of potential moderate to severe sleep apnoea.
The sleep apnoea feature will be available in 150 countries following FDA approval. It will be accompanied by existing health features such as Afib alerts, cardio fitness, and the ECG app from previous Apple Watch models.